
Valence electron pairs are being shared between the carbon atoms to create a single carbon-carbon bond. This is why atoms, and also chemical compounds, lose or gain electrons to become ions, and also why they form ionic and covalent bonds.įigure 2: Depiction of the covalent sigma bonds in Ethane.
PERIODIC TABLE VALENCE ELECTRONS FULL
In general, atoms want to have full valence electron shells. Noble gases neither want to gain or lose an electron, which means they tend to be chemically inert (unreactive). Noble gases are elements that have a full valence shell, meaning that the outer shell is completely filled with electrons. Thus, it wants to pick up an electron and become a Cl - ion. This can be seen with Chlorine, which in it's neutral state is missing one electron in it's valence electron shell. An atom can also gain an electron (usually to fill it's valence shell) and turn into a negatively charged ion. Thus it wants to lose a single electron and become an Na + ion. For example, sodium (Na) has one electron in it's outer shell. When an electron leaves a neutral atom, it loses a negative charge and turns into a positively charged ion. This can be to create an ionic bond or to become an ion.
PERIODIC TABLE VALENCE ELECTRONS FREE
In chemical reactions, the electrons can even break free from the valence shell. This means that electrons in the inner shells can absorb bits of energy and move (jump) to the valence electron shell. In addition, core electrons in the inner shells have lower energy levels than the valence electrons occupying the outer shell. This difference comes from the electric force being an inverse square law. Valence electrons are the farthest from the positive charge (the protons) and thus tend to be easier to remove than core electrons this means that it takes them less energy to move far away from the atom. Electrons that are closer to the nucleus are in filled orbitals and are called core electrons. Valence electrons are the electrons orbiting the nucleus in the outermost atomic shell of an atom. Each of these orbitals serves to create a shell of electrons in the atom. These orbitals and the energy needed to remove each of these electrons from the atom are set by quantum mechanics. This picture does not address the quantum mechanics of electrons around atoms.Įlectrons exist in orbitals around a nucleus.

The valence electrons in a molecule can be represented as the bonding electrons or non-bonding electrons (lone pairs).Figure 1: The two yellow electrons on the outermost oval are the valence electrons the other 10 electrons are core electrons. Note: The property of the valence electrons are that they are the only electrons which take part in the chemical bonding. Example: elements present in 1 st group hydrogen, lithium, sodium have the same number of valence electrons that is 1. As we move from left to right across the periods in the periodic table the number of valence electrons increases.Īcross the group, the number of valence electrons remains the same which shows that the chemical elements present in the same group have the same number of valence electrons. The valence electrons vary across the periodic table. The valence electrons are the electrons which are present in the outermost orbital of the electronic configuration. The atomic number of the chemical elements represents the number of electrons present in the atom of the element.
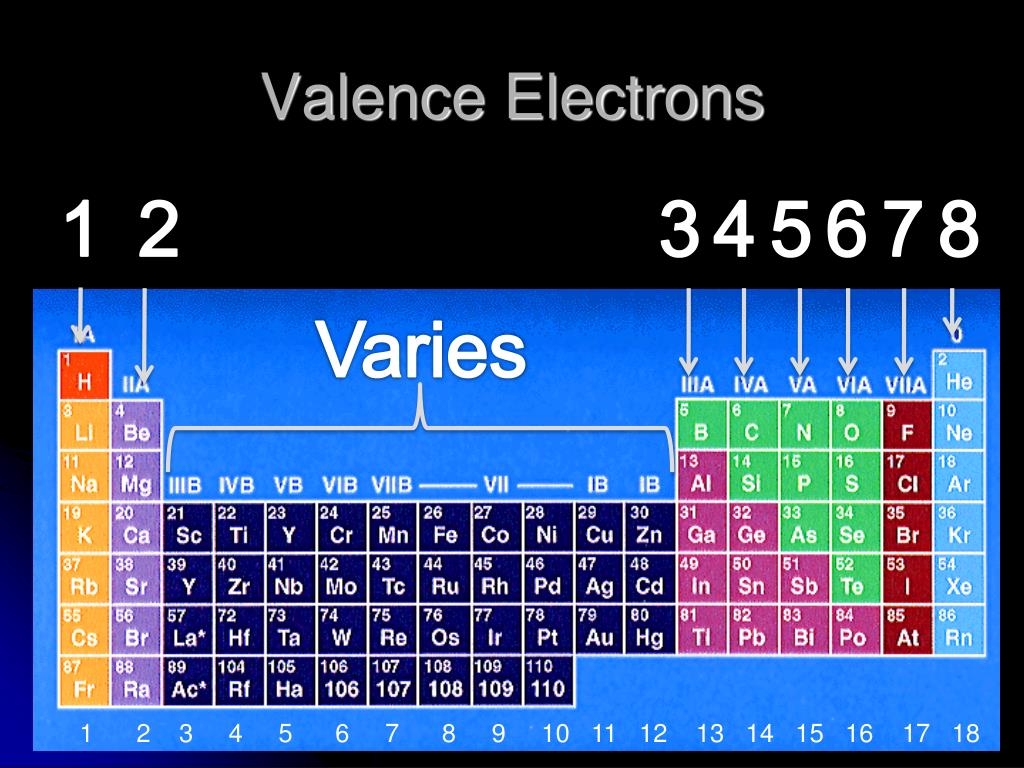
As stated in the law, the atomic number of the chemical element is related to the physical and chemical behavior of the chemical element due to this reason the chemical element shows periodicity in their physical and chemical behavior. In the modern periodic table the chemical elements are arranged according to their atomic number which keeps increasing as we move in the periodic table.

Moseley discovered the Modern Periodic law and states that the physical properties and the chemical properties of the chemical elements are the periodic function of their atomic number. The valence electrons are present in the outermost orbital of the chemical element.

Hint: The atomic number of the chemical element represents the number of electrons present in the atom of the chemical element.


 0 kommentar(er)
0 kommentar(er)
